PRODUCT PORTFOLIO
Symphony™
Bluejay Diagnostics is pleased to present Symphony, a near-patient biomarker detection platform. Symphony aims to improve healthcare outcomes by providing quantitative clinical chemistry results, vital for triage and acute care situations.[1] Symphony combines modern advances in interrupted microfluidics, nanotechnology, and enzyme linked immunosorbent assays (ELISA) to enable clinical chemistry results faster by bringing testing near patients.[1]
Symphony's design promotes usability by eliminating specialized trainings and infrastructure. Symphony's compact, and cost-effective design is enabled by the single-use Symphony Cartridges, which includes all reagents and integrates whole blood processing, precision ELISA of specific biomarkers, and waste handling. Simply add a few drops of blood into the Symphony Cartridge, insert the Symphony Cartridge into Symphony Analyzer, and in ~20 minutes interpret the results.
FEATURES AND BENEFITS
-
Compact, portable, and cost-effective.
-
No additional equipment required.
-
Simultaneously test up to six samples or six biomarkers.
-
Results in ~20 minutes.
-
Quantitative and recording in pg/mL.
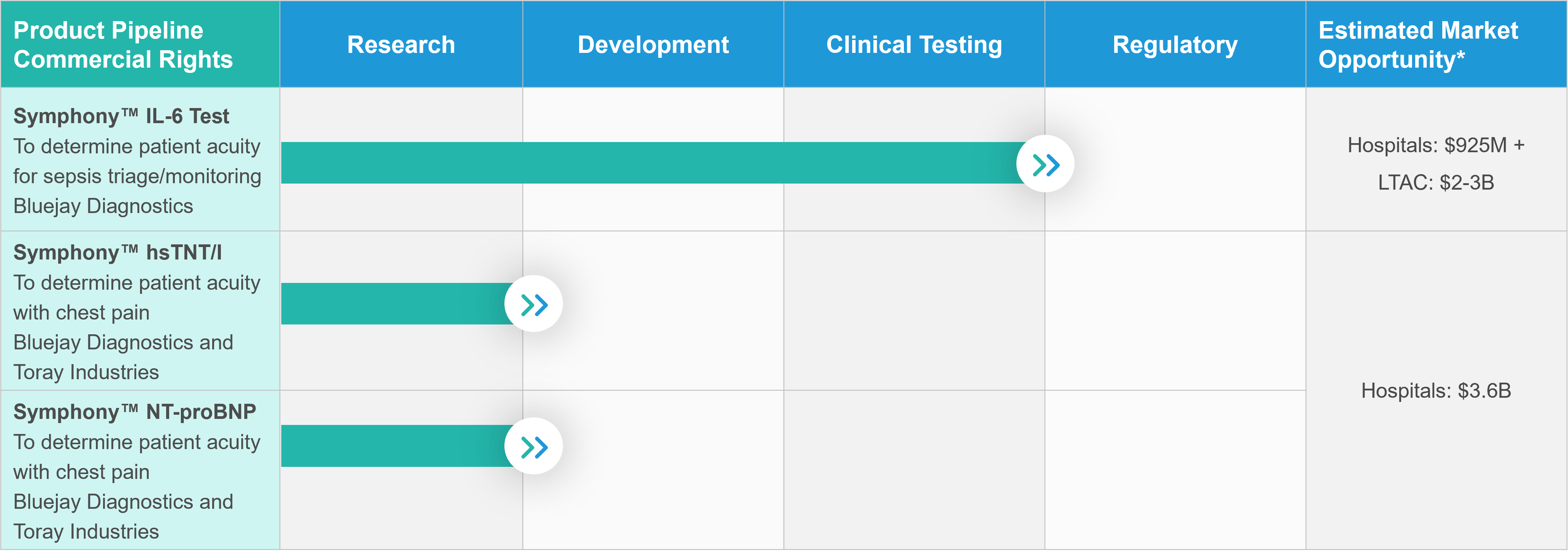
SYMPHONY™ PRODUCT PIPELINE
SYMPHONY ANALYZER SPECIFICATIONS
-
Dimensions: Width 30.0 cm (11.8 in.), Depth 42.0 cm (16.5 in.), Height 32.5 cm (12.8 in.)
-
Weight: 16 kg (35 lbs)
-
Power: 120 Vac (Nominal), 50/60 Hz
-
Calibration: Factory – electronic, mechanical, thermal
-
Display: LCD Touchscreen
-
Operating Temperature: 15-28°C (59-82°F)
-
Transport/Storage Temperature: -10-60°C (14-140°F)
-
Onboard Thermal Printer
-
Barcode Reader (Optional)
SYMPHONY ANALYZER IN ACTION
NOTES
[1]. Symphony is currently for Research Use Only and these statements have not been evaluated by the FDA. This product is not intended to diagnose, treat, cure, or prevent any disease.

